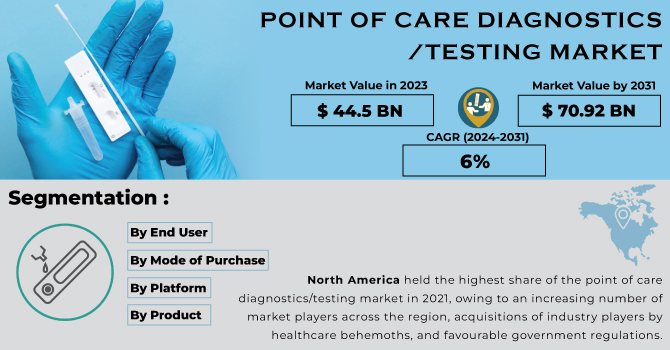
The global Point of Care (POC) Diagnostics Market, valued at USD 44.5 billion in 2023, is poised for significant growth. According to recent projections, the market is expected to reach USD 70.92 billion by 2031, growing at a compound annual growth rate (CAGR) of 6% over the forecast period from 2024 to 2031. This substantial growth underscores the increasing demand for rapid and convenient diagnostic testing, driven by advancements in technology, rising prevalence of chronic diseases, and the need for efficient healthcare delivery.
Understanding Point of Care Diagnostics
Point of Care (POC) diagnostics refers to medical diagnostic testing conducted at or near the site of patient care. POC testing provides immediate results, enabling faster clinical decision-making and improved patient management. These tests are commonly used in various settings, including hospitals, clinics, ambulatory care, and home care, covering a wide range of applications such as blood glucose monitoring, infectious disease testing, cardiac markers, and pregnancy testing.
Get a Free Sample Report of Point of Care Diagnostics/Testing Market: https://www.snsinsider.com/sample-request/2596
Market Drivers
Several key factors are driving the growth of the POC diagnostics market:
- Technological Advancements: Continuous advancements in diagnostic technologies, such as microfluidics, biosensors, and lab-on-a-chip, are enhancing the accuracy, speed, and convenience of POC testing. These innovations are driving market growth by expanding the range of tests available at the point of care.
- Rising Prevalence of Chronic Diseases: The increasing prevalence of chronic diseases, such as diabetes, cardiovascular diseases, and infectious diseases, is fueling the demand for rapid diagnostic testing. POC diagnostics enable timely detection and management of these conditions, improving patient outcomes.
- Growing Need for Rapid Testing: The need for rapid and accurate diagnostic testing has become more critical in recent years, especially in the wake of the COVID-19 pandemic. POC tests provide quick results, facilitating prompt treatment and reducing the burden on healthcare systems.
- Convenience and Accessibility: POC diagnostics offer the convenience of testing at or near the site of patient care, reducing the need for laboratory visits and minimizing turnaround times. This accessibility is particularly beneficial in remote and underserved areas, improving healthcare delivery.
- Focus on Preventive Healthcare: There is a growing emphasis on preventive healthcare and early diagnosis to reduce healthcare costs and improve patient outcomes. POC diagnostics play a crucial role in early disease detection and monitoring, supporting preventive healthcare initiatives.
Challenges and Opportunities
While the POC diagnostics market holds significant promise, it also faces several challenges:
- Regulatory and Quality Issues: Ensuring the accuracy and reliability of POC tests is critical, as diagnostic errors can have serious consequences. Stringent regulatory requirements and quality control measures are essential to maintain high standards of testing.
- Cost and Reimbursement Issues: The cost of POC diagnostic devices and tests can be a barrier to adoption, particularly in low-resource settings. Ensuring adequate reimbursement policies and developing cost-effective solutions are crucial to overcoming this challenge.
- Integration with Healthcare Systems: Integrating POC testing with existing healthcare systems and electronic health records (EHR) can be complex. Seamless integration is essential for efficient data management and improved patient care.
Despite these challenges, the market presents numerous opportunities:
- Expansion into Emerging Markets: Expanding the adoption of POC diagnostics in emerging markets presents a significant growth opportunity. These regions are experiencing rapid advancements in healthcare infrastructure and increasing demand for accessible diagnostic testing.
- Development of Multiplex Tests: Developing multiplex tests that can simultaneously detect multiple biomarkers or pathogens in a single assay can enhance the efficiency and utility of POC diagnostics. Investment in research and development can drive innovation in this area.
- Collaborations and Partnerships: Collaboration between diagnostic companies, healthcare providers, and research institutions can drive innovation and improve the quality of POC tests. Strategic partnerships can expand market reach and provide comprehensive diagnostic solutions.
Key Market Players
Several key players are driving innovation and growth in the POC diagnostics market:
- Abbott Laboratories: Abbott offers a comprehensive range of POC diagnostic tests and devices, including glucose monitoring, infectious disease testing, and cardiac markers. Their advanced technologies and global reach ensure efficient service delivery.
- Roche Diagnostics: Roche provides innovative POC diagnostic solutions that leverage cutting-edge technology to enhance patient outcomes. Their commitment to quality and customer satisfaction ensures reliable service delivery.
- Siemens Healthineers: Siemens offers a wide range of POC diagnostic tests, including blood gas analyzers, coagulation monitors, and infectious disease assays. Their focus on research and development drives market growth.
- Danaher Corporation: Danaher provides advanced POC diagnostic solutions that optimize testing processes and improve patient outcomes. Their integrated platform supports value-based care models and enhances service efficiency.
Future Outlook
The future of the POC diagnostics market looks promising, with continued advancements in technology and increasing demand for rapid and convenient testing driving growth. As healthcare organizations strive to improve patient outcomes, reduce healthcare costs, and ensure timely diagnosis and treatment, the adoption of advanced POC diagnostics is expected to accelerate.
The projected growth to USD 70.92 billion by 2031 reflects the transformative potential of these tests in modern healthcare systems. With a CAGR of 6% over the forecast period, the market is poised for substantial growth, offering innovative solutions that could revolutionize diagnostic testing and patient care.
Conclusion
In conclusion, the POC diagnostics market is on a trajectory of significant growth, driven by technological advancements, rising prevalence of chronic diseases, growing need for rapid testing, convenience and accessibility, and focus on preventive healthcare. While challenges remain, the opportunities for expansion and innovation are vast. The projected market size of USD 70.92 billion by 2031 underscores the substantial impact POC diagnostics are set to have on the future of healthcare.
Other Reports You May Like:
Growth Hormone Deficiency Market Size
Pharmaceutical Excipients Market Size
Real-Time PCR (qPCR) Market Size